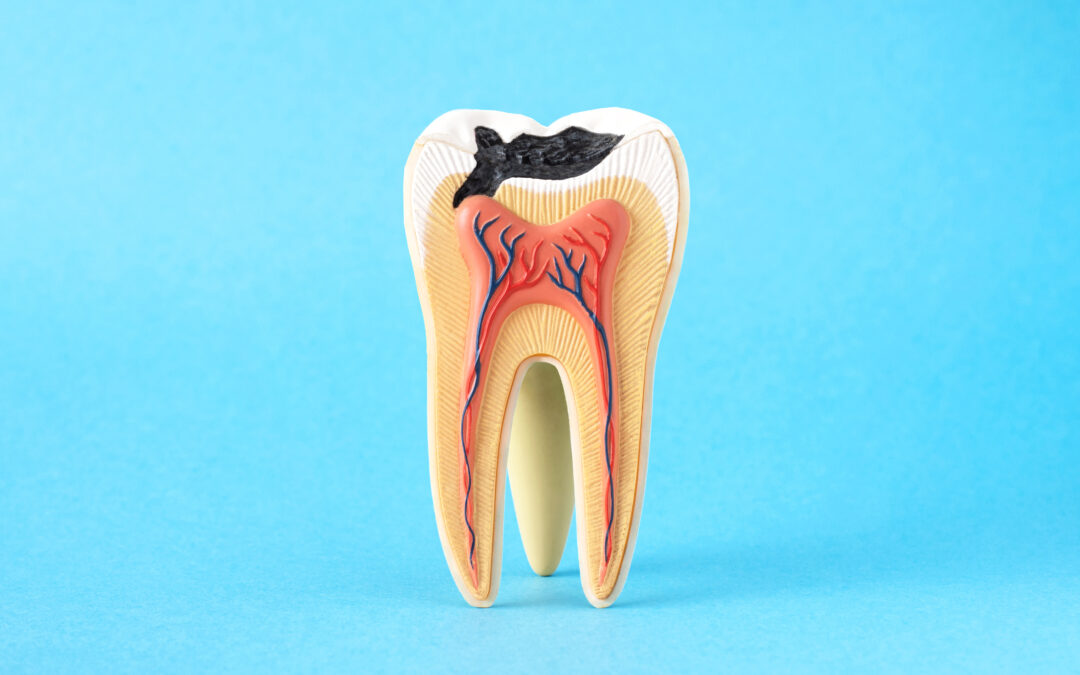
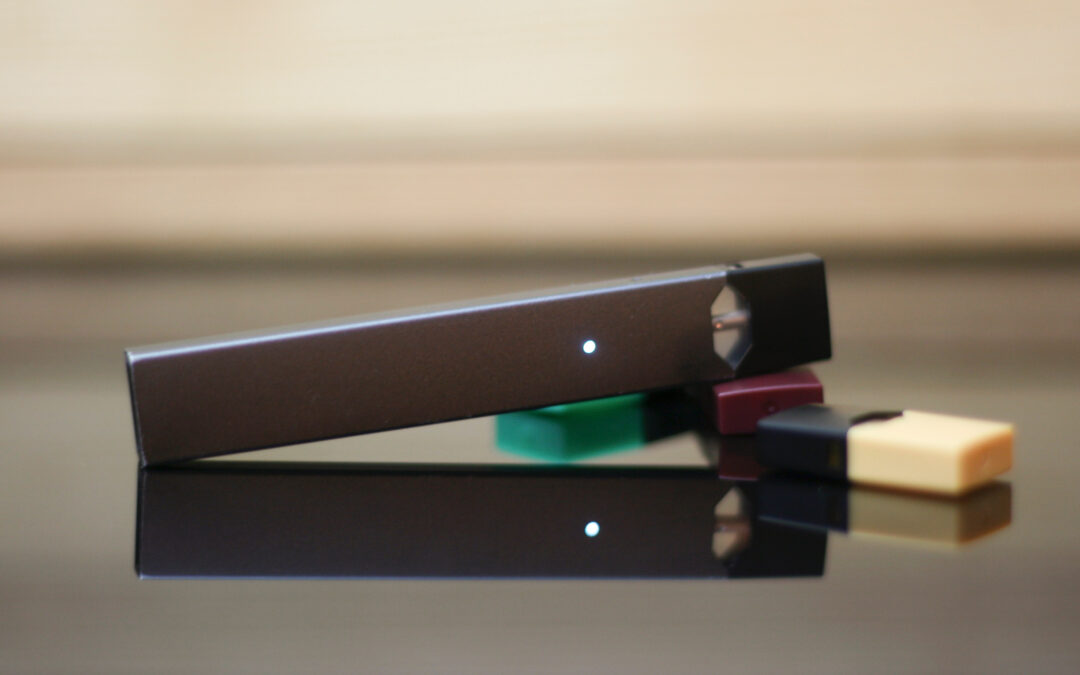
In the realm of mass tort litigation, managing vast datasets and extracting meaningful insights are paramount for law firms. Verus provides an elite internal data team dedicated to elevating your data collection, management, and analysis to the next level. By working...